Abstract
Background: Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is currently the standard of care for diffuse large B-cell lymphoma (DLBCL). Although heterogeneous, approximately 60 % of patients are cured while 10% of patient's refractory to initial chemotherapy. These outcomes are highly dependent on the dose intensity of chemotherapy received with poor outcomes associated with reduced dose intensity. Febrile neutropenia is one of the most common adverse events experienced leading to dose delays and interruptions. Therefore, we planned to evaluate the incidence of febrile neutropenia, its influence on delaying chemotherapy doses, and the need for subsequent growth factor support. We also planned to identify risk factors for the development of febrile neutropenia after the initiation of chemotherapy.
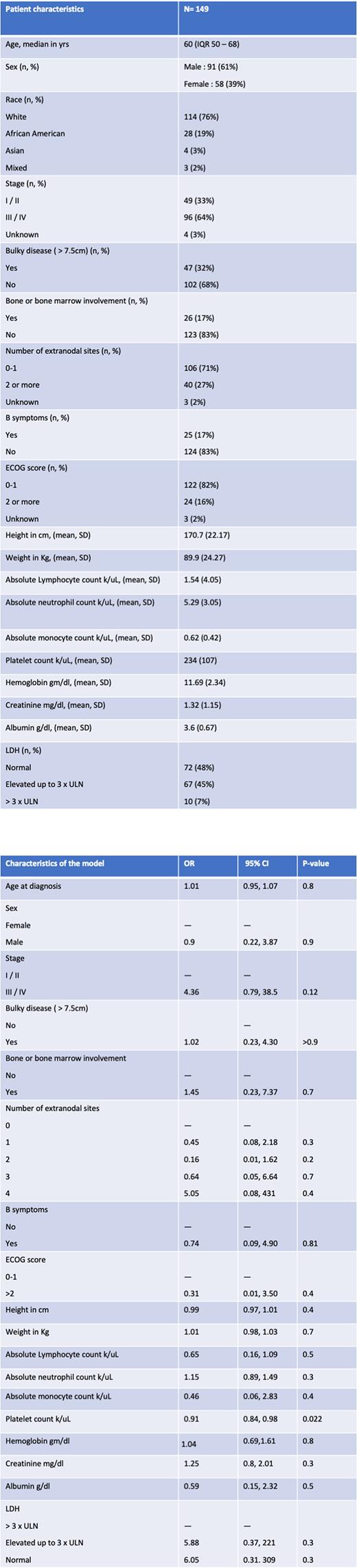
Methods: We conducted a single-center retrospective study including adults with newly diagnosed DLBCL initiating R-CHOP-21 chemotherapy without growth factor support between dates January 2016 to February 2021 at the University of Alabama at Birmingham. We extracted baseline clinical and demographic variables (age, sex, race, stage, bulky disease > 7.5 cm, number of extranodal sites, osseous or bone marrow involvement, presence of B-symptoms, ECOG score, height, weight) and laboratory variables (absolute lymphocyte count, absolute neutrophil count, absolute monocyte count, platelets, lactate dehydrogenase, albumin, and creatinine) within 7 days of initiating treatment. The outcome variable was the development of febrile neutropenia during treatment. We used a multivariate regression model to identify risk factors for the development of febrile neutropenia adjusted for demographic and laboratory covariates listed above. Collinearity amongst variables was evaluated and highly correlated variables were excluded. The hypothesis testing was two-sided, and the level of significance was chosen as 0.05.
Results: Of the 257 patients with a diagnosis of DLBCL, 149 patients met the inclusion criteria and were included in our cohort. The median age at diagnosis was 60 yrs (IQR 50 - 68) with 61% male and 77% white. Stage I/II was present in 33% of patients with bulky disease in 32% pts and 17% with involvement of the bone or bone marrow at diagnosis. The ECOG PS was less than 2 in 82% of patients with only 17% of patients endorsing B symptoms prior to treatment. Delays in treatment by more than 3 days were seen in 49 pts (32%) and 32 pts(21%) had delays of more than 7 days. Treatment discontinuation due to toxicity or death was seen in 19 pts (13%). Febrile neutropenia was experienced by 17 pts (11%) requiring subsequent growth factor support in 8 pts and leading to subsequent delays in treatment in all but one patient. Increasing platelet count (Odds Ratio, OR 0.91; 95%CI 0.84 - 0.98; p value 0.02) was associated with a decreased risk of developing febrile neutropenia during treatment.
Conclusions: R-CHOP chemotherapy is generally well-tolerated, however, febrile neutropenia remains the most-common toxicity occurring in approximately 11% of patients and leads to associated treatment delays. Lower platelet count prior to initiation of chemotherapy was identified as a risk factor for developing febrile neutropenia adjusted for other known variables of bone marrow function. Therefore, future predictive models of febrile neutropenia in DLBCL should include baseline platelet count as a feature.
Affiliations:
1. The Joe R. and Teresa Lozano Long School of Medicine, San Antonio, TX
2. Department of Medicine, University of Alabama at Birmingham, Birmingham, AL
3. Mays Cancer Center, UT San Antonio, San Antonio, TX
4. Division of Hematology and Oncology, Department of Medicine, O'Neal Comprehensive Cancer Center, University of Alabama at Birmingham, Birmingham, AL
Disclosures
Narkhede:Genmab: Research Funding; Genetech: Research Funding; Roche: Research Funding; Gilead: Research Funding; Gilead/Forty-seven: Research Funding; EUSA pharmaceuticals;: Research Funding; Seagen Inc.: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal